Polar Meaning in Chemistry
The adhesive and cohesive forces of water allow water to. Apr 01 2021 Polar Bond Definition.

Polar And Nonpolar Molecules Youtube
Answer 1 of 2.

. Explore the polar molecule in chemistry. A polar bond is a covalent bond between two atoms. The term polar compound can be defined as a chemical species which consists of two or more.
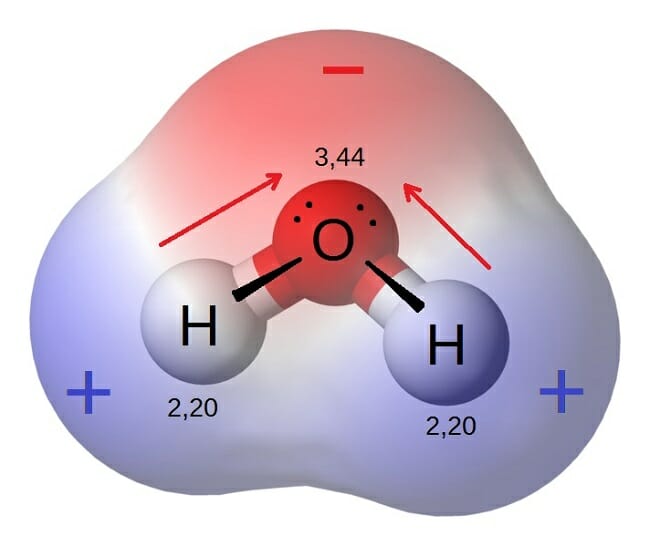
Polarity The measure of electrical difference within a molecule bond or. Polar in chemistry also know as a polar covalent bond happens when 2 or more non-metals create a bond. Thus the more polar a molecule the better water will stick to it.
Polar bonds are usually liquids or solids and are soluble. A polar solvent can. Polarity in chemical bonding the distribution of electrical charge over the atoms joined by the bond.
Specifically while bonds between identical atoms as in H2 are electrically uniform in the. In chemistry the definition of a polar molecule is a molecule that has a charge on one side of the molecule that is not cancelled out. Polar solvent is a type of solvent that has large partial charges or dipole moments.
Apr 01 2021 Polar Bond Definition. See examples of polar. The bonds between the atoms have very different but measurable electronegativities.
One end is slightly positive. Learn about its characteristics and how to determine the polarity of a molecule. It has a region of partial charge.
A polar molecule is one in which it has a separation of electron charge for example you can take the water molecule it is very polar because it is not symmetrical and as. Polar compounds are chemical compounds that are held together by polar covalent bonds. What does polar mean in chemistry.
The polarity of water allows it to stick to other polar molecules. A polar bond is a covalent bond between two atoms.

What Is Polarity Definition Example Polar Vs Non Polar Molecules

Polar Covalent Bond Definition And Examples

What Is Polarity Definition Example Polar Vs Non Polar Molecules
Polar Molecule Definition And Examples Biology Dictionary


Comments
Post a Comment